The Englander Institute for Precision Medicine (EIPM) Research Facility is committed to driving research forward to bring the right treatment to the right patient at the right time. We actively work with clinical and research departments with the goal of bringing new precision medicine technologies to all patients. Our multidisciplinary team drives genomics-based care, and channels efforts towards developing predictive models and new therapies where genomic data can offer hope and transform health care.
Our unique precision medicine platforms scale beyond single investigator laboratories to foster collaborations that fuel discovery and advance treatments to patients. We aid in comprehensive project management and research consultation to aid investigator-led studies. We request that researchers acknowledge the Englander Institute for Precision Medicine (EIPM) of Weill Cornell Medicine in publications, abstracts, and presentations when utilizing our resources.
For information regarding our collaborative services and resources, please read below about each Facility group for further details.
For more information about our research initiatives and platforms, please visit our website.

The EIPM Research Laboratory serves as the central repository for banked oncology specimens and the sample processing hub for downstream sequencing efforts from several ongoing clinical trials with many samples coming from EIPM collaborative research efforts with clinical WCM sites. We are focused on targeted biobanking in areas of oncology research, specimen processing, precision pathology, & comprehensive genomic/transcriptomic testing of patient’s blood and/or tissue to help address key outstanding questions vital to defeating disease. Finally, we aid in comprehensive project management and research consultation to aid investigator-led studies. Please contact Michael Sigouros (mis2092@med.cornell.edu) if you are interested in exploring a new translational research collaboration prior to submission of a project to assess feasibility.
The laboratory is equipped with several specimen processing and handling instruments: three Eppendorf 5424R centrifuges, two Eppendorf 5810R centrifuges, a Promega AS4500 Maxwell(R) RSC DNA and RNA extraction instrument, an Agilent TapeStation 4200 DNA QC instrument, and several Qubit Fluorometers (Qubit 4 & Qubit Flex). The lab utilizes two Hamilton STAR automated liquid handlers for high throughput sample preparation for large scale research programs: the Hamilton easyBlood STARlet robot is designed for automated fractionation of blood specimens and the Hamilton Extraction STAR is configured for automated extraction of DNA and RNA from various specimen types such as tissue, blood, cells, and saliva. For pathology and imaging applications, the lab is equipped with a Leica Aperio AT2 Digital Slide Scanner and several microscopes: 2 Olympus BX43 and 1 Olympus CX41.
The EIPM Lab offers below laboratory processing services for collaborative projects:
Sample extractions are commonly requested in conjunction with genomic projects and clinical trials from WCM investigators. Project-based sample extraction will now be offered to the WCM community and beyond. This can be requested by following the instructions on project consult on the main page.
*Note: EIPM extraction protocols have been validated across several different solid tumor cohorts/studies. DNA and/or RNA sample quantity and quality may be variable based on parent sample processing/preservation methods. EIPM cannot guarantee that samples will generate the required amount of DNA and/or RNA needed for particular assay or downstream application. Please proceed at your own risk
At EIPM, the Hamilton Extraction STAR can perform high throughput DNA or RNA extractions from a variety of starting materials. Please see below for links to comprehensive QC documentation for our validated protocols.
Using NeoGeneStar’s Kit, we have validated automated extractions of cfDNA from plasma. Using Omega Mag-Bind® Blood & Tissue DNA HDQ 96 Kit kits, we have validated extraction on the Star robot for the following sample types: Saliva, Cell Pellets, Buffy Coat, Frozen Tissue.
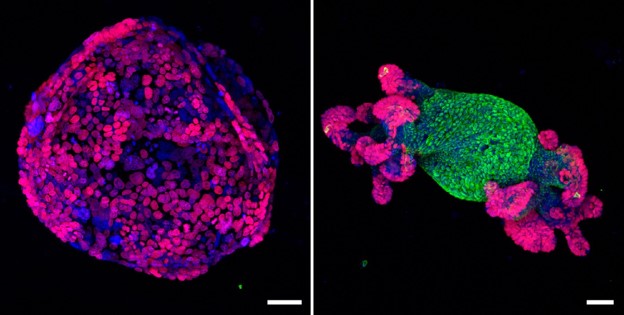
The EIPM develops organoids, which are patient-derived models, that are used to study disease progression and develop effective drug treatments. These miniature three-dimensional cellular structures are grown from a patient’s tumor sample and are highly useful in studying how different cancers develop, change, and might respond to various treatment options.
Organoids can serve as an ideal model to enable discoveries of novel therapeutic approaches which can be assessed via scientific research and in clinical trials and provide personalized therapeutic options for individual patients where standard clinical options have been exhausted.
Our platform is unique because we have advanced the technology to derive organoids from many different types of cancer, and only a few institutions across the country and around the world have done this.
The team develops organoids from metastatic and primary anatomic sites, obtained through biopsies, surgical resections, and rapid autopsy procedures. A critical step in successfully generating patient-derived organoids is validating their biological similarity with the primary tumor.
To this end, we characterize each patient-derived organoid with genomic sequencing, RNA seq, and histopathology. Validation of these models is achieved by comparison of DNA mutational profiles (ploidy, concordance, correlation of variant allele frequencies, mutational signatures) as well as RNA sequencing data through cluster analysis of the original tumor in comparison with the organoids.
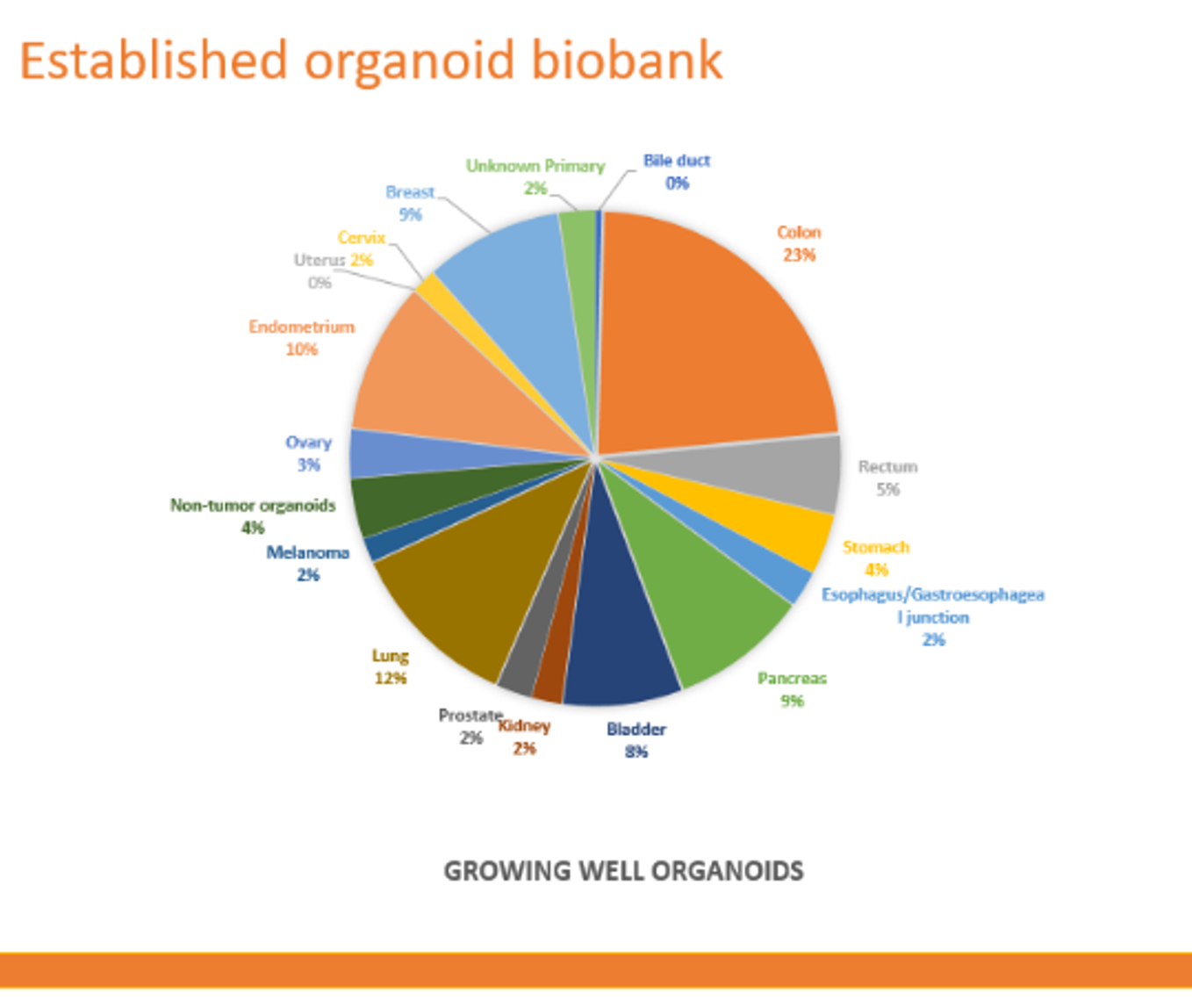
We now have a pan cancer biobank of more than 150 models which have been fully characterized via sequencing and/or pathology review and are now available for research use.
At the EIPM we strive to be at the forefront of translational research. We’ve recently paired the Helios™, mass cytometry by time-of-flight (CyTOF) instrument with the Hyperion imaging module. With this advanced technology for single-cell analysis, we are providing investigators with a powerful, affordable and actionable tool for exploring complex biological processes.
The mission of our core is to provide state-of-the-art technology and services to assist Weill Cornell scientists and physicians in designing and conducting cutting-edge immunology research and translational studies using precious patient samples for high-impact discoveries for the treatment of human diseases. We collaborate with multiple core facilities at Weill Cornell while developing new additional platforms needed.
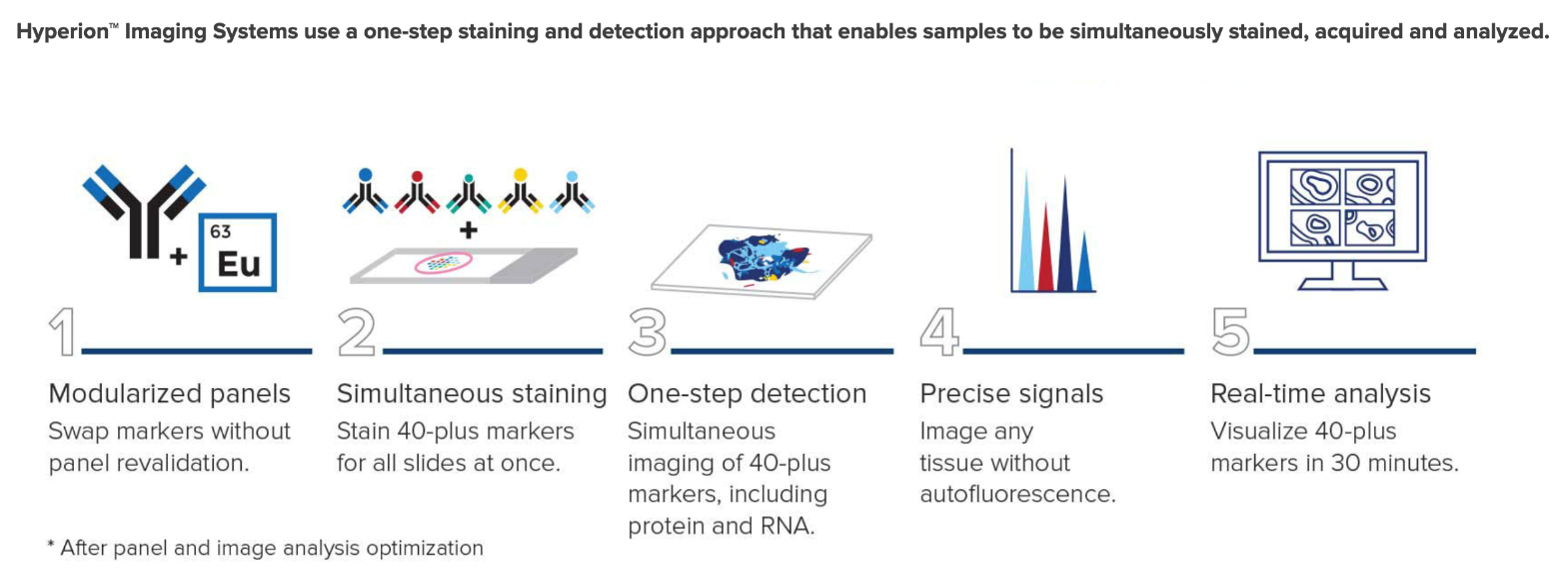
The Hyperion™ Imaging System is a mass cytometry-based high-resolution laser ablation system that allows for the highly multiplexed imaging with up to 135 channels available. The system is designed to detect metal-tagged antibodies bound to the cell surface and intracellular proteins in tissue sections using immunohistochemical methods. The system allows for high-resolution cellular profiling in spatial proximity, enabling detection of disease cells and immune cell populations within the context of the tissue structure.
Imaging Mass Cytometry employs laser ablation of tissue sections stained with a leap in dimensionality of 40 markers simultaneously, generating millions of pixels with a resolution comparable to light microscopy but with high content of mass cytometry. This technology unveils the interaction between cell types in their tissue context and throws more light into the characterization of functional and signaling state of the cells.
CyTOF aids in multiplexing and deep profiling of single cells and is expected to have significant clinical applications. With this Inductively Coupled Plasma (ICP) – Time of Flight-based technology, one can profile 40 markers at a time on a single cell and learn more about the functional and phenotypic relevance of the cell state at a single cell resolution.
|
Tissues analyzed |
|
FFPE, frozen tissues, biopsies |
|
Targets analyzed |
|
Proteins and RNAs |
|
Tissue thickness (full ablation) |
|
≤7 µm |
|
Addressable sample size |
|
≥16 mm x 55 mm |
|
Scan area |
|
≥1 mm2/ 4 Hr (@100Hz) |
|
Ablation spot size |
|
≤20 % |
|
Optimal operating temperature |
|
21 ± 1 °C |
|
Workflow |
|
Applications |
|
Frame relevant research goal |
|
Immuno-phenotyping |
|
Identify markers that augment research goal |
|
Functional and Signaling state of a cell in tissue context |
|
Design the panel |
|
Cytokine/Chemokine expression |
|
Antibody validation |
|
Normal vs. Diseased state comparison to identify any clinically significant cell transformation |
|
Antibody Titration |
|
Proliferation analyses |
|
Staining and scanning on Hyperion |
|
Apoptosis and Necrosis profiling |
|
Analysis of the data (cell segmentation, ViSNE plot, etc.) |
|
|
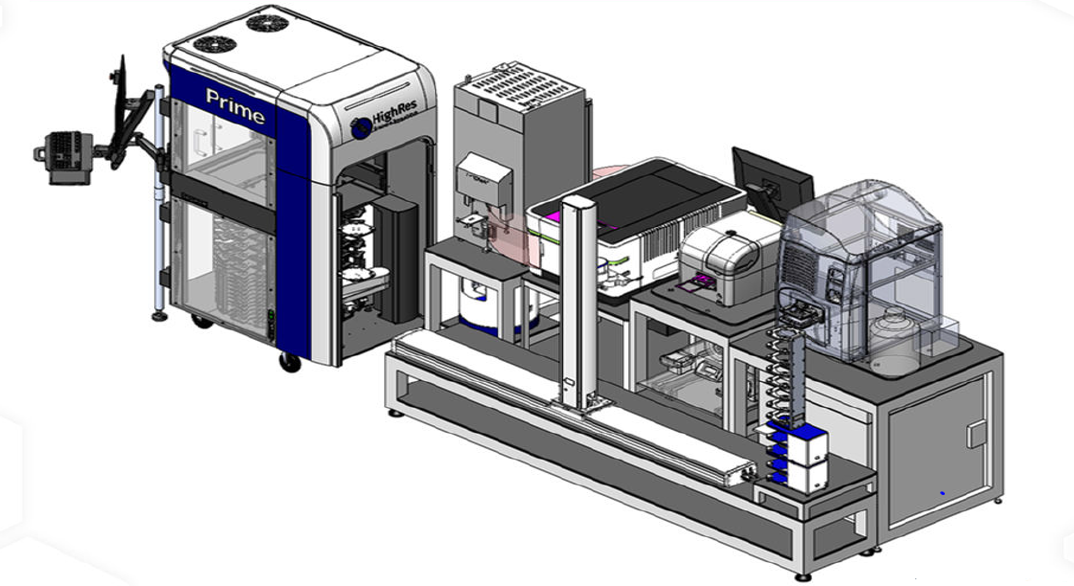
The High Throughput Drug Screening (HTDS) Facility provides professional HTDS services to researchers internally and externally. The service includes screening of compounds in 2D or 3D cell lines, assay development, experimental design, sample manipulation, instrumentation, data analysis, interpretation, and validation. The HTS Platform is an integrative robotics system that consist of HighRes BioSolutions Prime automated liquid handler, in-house static and dynamic incubator systems, and Operetta CLS High Content Imaging System connected by two robotic arms and a powerful scheduling software. We provide an FDA approved drug library, anti-cancer library, kinase inhibitor library, and routinely prepare custom libraries for single point, dose-response, and combinatorial screening.
CDD Vault
A powerful web-based database management and analysis system, the Collaborative Drug Discovery vault, will track usage of chemical libraries and performs data analysis to identify lead candidates, including data analysis, enabling rapid identification of potential hits.
Operetta CLS High Content Imaging System
The Operetta CLS high-content analysis system has all the speed, sensitivity, and resolution to characterize sub-cellular details and quantitative cellular analysis of fixed or live cells.

| Michael Sigouros | Senior Research Operations Manager | (646) 962-7536 | mis2092@med.cornell.edu | Research Laboratory |
| David Wilkes | Hamilton Platform Manager | (646) 962-6189 | dcw2001@med.cornell.edu | Research Laboratory |
| Olivia Baldanza | Laboratory Administrative Assistant | (646) 962-8705 | olb4004@med.cornell.edu | Research Laboratory |
| Jenna Moyer | Organoid Platform Manager | - | jem4012@med.cornell.edu | Organoid |
| Hiranmayi Ravichandran | CYTOF Platform Manager | - | hir4001@med.cornell.edu | CYTOF |
| Ilkay Us | HTDS Platform Manager | (212) 746 6464 | ilu2001@med.cornell.edu | HTDS |
| Michael Sigouros | Senior Research Operations Manager | (646) 962-7536 | mis2092@med.cornell.edu |
| David Wilkes | Hamilton Platform Manager | 646-962-6189 | dcw2001@med.cornell.edu |
| Olivia Baldanza | Laboratory Administrative Assistant | (646) 962-8705 | olb4004@med.cornell.edu |
| Jenna Moyer | Organoid Platform Manager | - | jem4012@med.cornell.edu |
| Hiranmayi Ravichandran | Director of Spatial Biology | - | hir4001@med.cornell.edu |
| Ilkay Us | HTDS Platform Manager |
| Hours | Location |
|
9 am - 5 pm |
413 East 69th Street |
| Name | Role | Phone | Location | |
|---|---|---|---|---|
| Olivia Baldanza |
Laboratory Administrative Assistant
|
646-962-8705
|
olb4004@med.cornell.edu
|
Belfer Research Building
|
| Available Services |
| ► EIPM Lab Processing - Other Lab Services (6) | |||
| Name | Description | Price | |
|---|---|---|---|
| Digitize Slides on AT2 | Inquire | ||
| eSlide Manager Access & Use | Inquire | ||
| Image Hosting | Inquire | ||
| Image Hosting Admin Fee | Inquire | ||
| Import Digital Slide Images |
This service includes importing H&E Slides from .svs format into Aperio eSlide Manager. |
Inquire | |
| Transfer Slide Images To Other Storage |
This service includes transferring slides to a predetermined location:
|
Inquire | |